Master Data-Driven IDMP Compliance: Fresh Gravity’s Approach
October 11th, 2022 WRITTEN BY Neha Inamdar Director, Data Science & Analytics Tags: data management, IDMP, Life Sciences

Written By Neha Inamdar Director, Data Science & Analytics
IDMP Compliance – An opportunity for building Enterprise Data Assets
Biotech, pharmaceutical, and medical device companies need to conform to a plethora of guidelines and regulations, and often, find themselves responding to regulatory needs in more of a reactive vs. proactive manner. The Identification of Medicinal Products (IDMP) is one such regulation that requires all pharmaceutical companies that market their products globally, to ensure that all product data submissions follow a uniform data structure with universal standards and vocabulary. The European Medicines Agency (EMA) is pioneering the implementation and enforcement of IDMP standards using its SPOR (Substance, Product, Organization, and Referential) framework. By early 2023, pharmaceutical companies are expected to begin submitting their product data to the EMA using IDMP-compliant web-based forms.
For an organization to be IDMP compliant, which means adhering to the structures and data attributes as outlined by guidance that the EMA has provided, there are data points required from a variety of different systems and product owners as outlined in the diagram below:
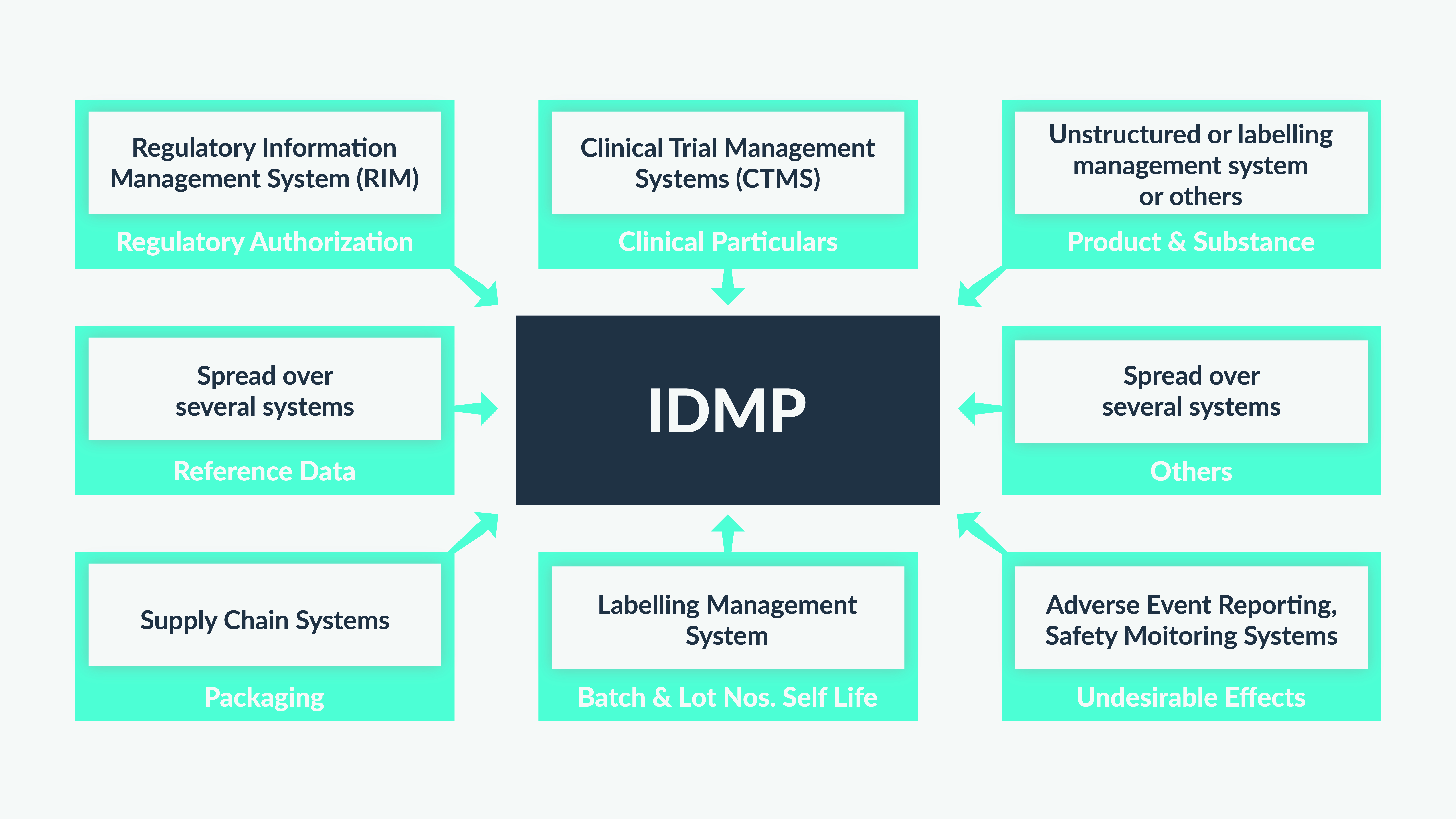 Figure 1: IDMP compliance necessitates the consolidation of data from multiple systems
Figure 1: IDMP compliance necessitates the consolidation of data from multiple systems
For a pharmaceutical company to comply with the IDMP standards, it is essential that business and IT stakeholders across R&D, Clinical, Supply Chain, Marketing, and Regulatory work together as a single cross-functional team and find ways to relate cross-functional data. This cross-functional data often does not conform to an enterprise-wide agreed-upon structure or definition and there is a lack of a common technical and business definition of the data. This leads to more efforts on manual curation to ensure that the most accurate and up-to-date data is submitted to regulatory authorities.
Master Data Management (MDM) solutions, by their very function, are well positioned to enable companies to bring together data from across multiple data sources, enforce standardization, and facilitate the creation of an acceptable enterprise-wide “golden record.” Data quality, governance, and management are the bedrock of an MDM solution and are also critical for the success of an IDMP initiative. Data governance puts into place controls and processes to drive better data quality and facilitate a more comprehensive understanding of data across the enterprise. It gives end-to-end visibility to all the data citizens, which includes data stewards, line-of-business users, data governance, and compliance teams. Data stewards can effortlessly locate data, understand its lineage and ownership and obtain comprehensive technical and business context for each data set.
Fresh Gravity’s Molecule to Market Solution
Fresh Gravity’s MDM-driven solution for IDMP compliance, Molecule to Market,
- Brings together data from multiple functions and systems thus allowing organizations to gain a much broader view of their interconnected data
- Lays the groundwork for breaking data silos between distinct functions of the pharma company (R&D, Regulatory, Manufacturing, Supply Chain, etc.)
- Allows the creation of a single version of truth for product data as it moves through the drug development lifecycle. Each stage in the lifecycle adds more details to the description of a medicinal product, and this can be effectively captured in an MDM system. Therefore, this solution is an opportunity to enable pharmaceutical companies to:
- Build a repository of Enterprise-wide Data Assets
- Identify opportunities for and improve Data Quality and Data Governance practices
- Create an Enterprise Architecture view for data across the Drug Development Value Chain and
- Improve Operational Efficiencies
More importantly, the MDM solution generates and traces the journey of a substance from drug discovery to commercialization and regulatory compliance and provides an enterprise-wide view of the data needed across the entire value chain.
Specifically, this solution brings to the table:
- An IDMP-compliant data model with “Medicinal Product” as the anchor entity, thereby ensuring that product data stays at the center of the relationships
- Integration with the EMA reference data service (SPOR RMS) for reference data standardization
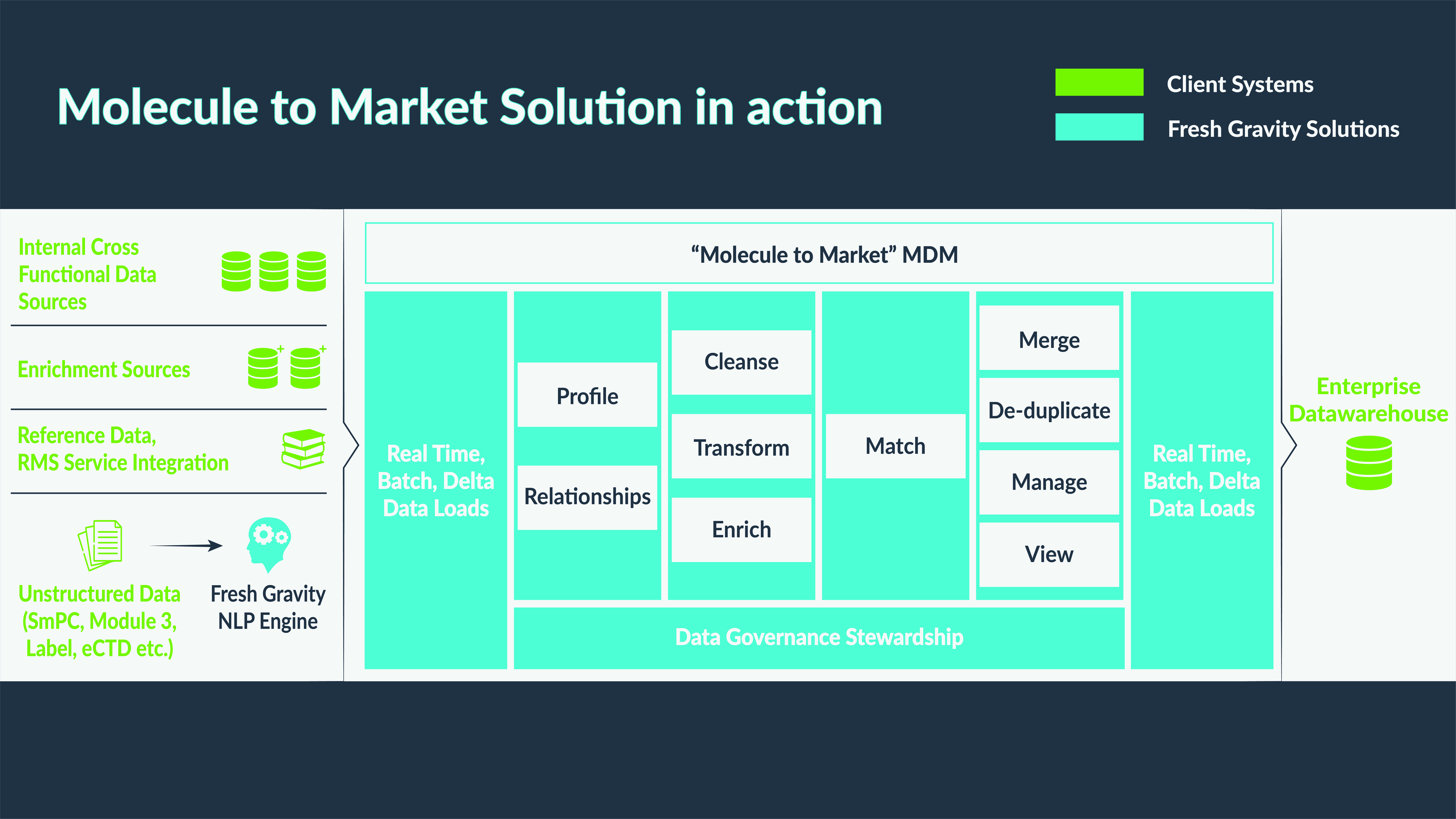 Figure 2: Molecule to Market Solution in Action
Figure 2: Molecule to Market Solution in Action
The working structure of the Molecule to Market solution
Data is ingested through the following data sources:
- Internal Cross-Functional Data
- Enrichment Sources: Additional enrichment sources such as Citeline, DrugDev, and DrugBank can be integrated with the Molecule to Market solution as per the business use case and requirements to add more details to data internal to pharma companies.
- Reference data and/or taxonomies may exist in different systems within the organization. These can be brought into the Molecule to Market solution to standardize the list of values utilized across the enterprise. Fresh Gravity has also developed a utility that uses API calls to populate all the reference data to mirror what is in EMAs SPOR Referential Management Service (RMS).
- Unstructured Data: There is IDMP-related data in unstructured formats across the organization in documents like SmPC, Module 3, eCTD, or the labeling documents. Fresh Gravity has built a Natural Language Processing engine to add an unsupervised learning method where relevant information can be extracted from unstructured data. Extracting data relevant to IDMP from unstructured data sources works as an accelerator that pushes data down into the MDM system.
Once data is brought into the MDM system, typical MDM functions take over, creating an entity/profile and building relationships between different entities. Thereafter, cleansing and, transformation rules are applied to the data, and match rules are applied to find potential matches within the data set. Data Stewards are then tasked with actioning merges to de-duplicate and manage the data from the UI. Once mastered, de-duplicated, and enriched data is ready, it can be sent to downstream data warehouses from where this data can be consumed by RIM systems for submission to the regulatory authorities.
Conclusion
As IDMP and similar directives include a variety of interactions with various health authorities over a span of time, Fresh Gravity recommends that pharmaceutical organizations opt for an iterative MDM solution. Fresh Gravity’s IDMP approach aims to build a robust MDM foundation that will assist in ongoing data quality maintenance and drive compliance and operational efficiencies in various areas for years to come.
For a demo of Fresh Gravity’s solution, or more information and questions, please write to neha.inamdar@freshgravity.com.
For more detailed information about this solution, please refer to the datasheet here.
.png)







